Lipid Dynamics during Mycobacteria Infection
Tuberculosis (Tb) is caused by Mycobacterium tuberculosis and remains one of the most deadly infectious diseases. The World Health Organization (WHO) estimates that in 2021, Tb killed 1.6 million people emphasizing the importance to develop new drugs, vaccines and diagnostic tools to reduce this burden in the future.
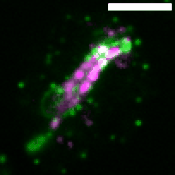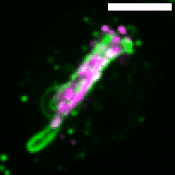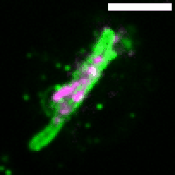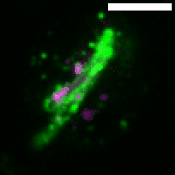
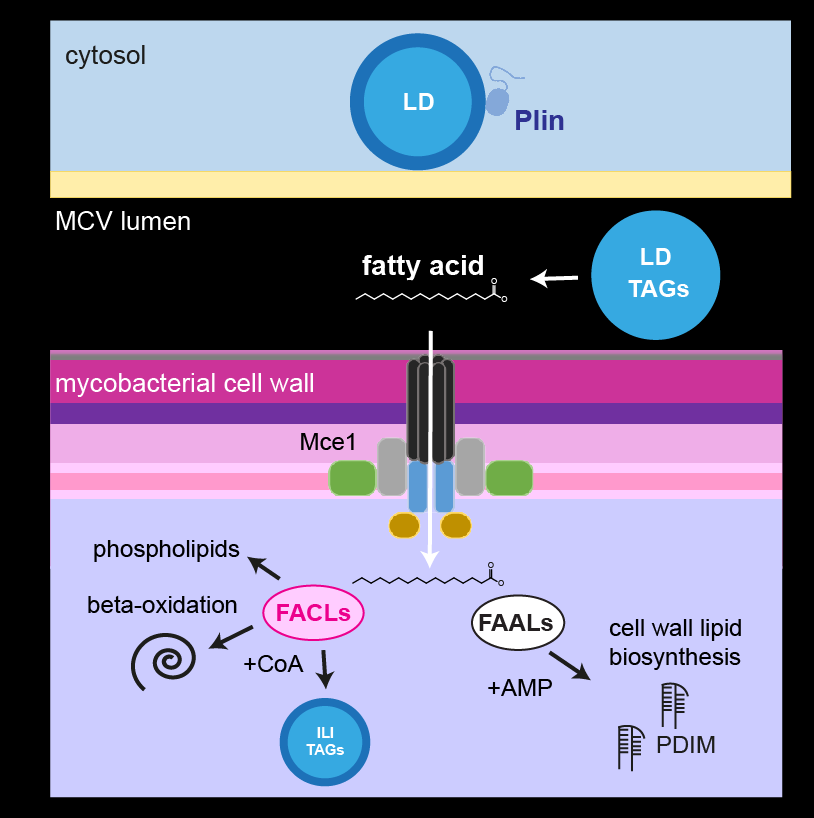
M. tuberculosis employs multiple strategies to survive intracellularly. One of its most striking adaptations is its ability to utilize host lipids such as fatty acids and sterols to: (i) generate energy, (ii) build its characteristic lipid-rich cell wall and (iii) produce storage lipids during infection. To be constantly in a fatty acid-rich environment, the pathogen actively contributes to generate the “foamy” phenotype in host macrophages, for which the accumulation of host lipid droplets (LDs) is characteristic.
Using the Dictyostelium discoideum/M. marinum infection system, we found that mycobacteria access host LDs to build up their own lipid storage organelles and exploit ER-derived phospholipids when LDs are lacking (Barisch et al., 2015; Barisch & Soldati, 2017). Moreover, we observed that mycobacteria that escaped from the Mycobacterium-containing vacuole (MCV) into the cytosol recruit LD-derived enzymes and regulatory proteins on their hydrophobic surface.
M. tuberculosis employs multiple strategies to survive intracellularly. One of its most striking adaptations is its ability to utilize host lipids such as fatty acids and sterols to: (i) generate energy, (ii) build its characteristic lipid-rich cell wall and (iii) produce storage lipids during infection. To be constantly in a fatty acid-rich environment, the pathogen actively contributes to generate the “foamy” phenotype in host macrophages, for which the accumulation of host lipid droplets (LDs) is characteristic.
Using the Dictyostelium discoideum/M. marinum infection system, we found that mycobacteria access host LDs to build up their own lipid storage organelles and exploit ER-derived phospholipids when LDs are lacking (Barisch et al., 2015; Barisch & Soldati, 2017). Moreover, we observed that mycobacteria that escaped from the Mycobacterium-containing vacuole (MCV) into the cytosol recruit LD-derived enzymes and regulatory proteins on their hydrophobic surface.
|
KEY INTEREST
The Barisch lab aims to unravel the molecular mechanisms by which pathogenic mycobacteria acquire lipids from their host to support chronic infection. Combining the application of functionalized lipid probes with mass spectrometry-based lipidomics and advanced microscopy techniques, the group analyses metabolic lipid flows between mycobacteria and their host at the subcellular and ultrastructural level.
|
Balancing Act: How a pertubation in fatty acid homeostasis impacts on vacuole escape of mycobacteria
|
To import fatty acids from their environment, mycobacteria are equipped with sophisticated transport machineries. However, the mechanism by which fatty acids are esterified with coenzyme A (“fatty acid activation”), an essential step for their further turnover, remains elusive. This project aims to characterize the function of fatty acid-activating enzymes in lipid synthesis and vacuolar escape of mycobacteria using the D. discoideum/M. marinum system. To characterize fatty acid flows and metabolism in host and bacteria mutants depleted in fatty acid-activating enzymes, a protocol that combines the use of bifunctional FA probes with expansion microscopy and lipidomics is established. - This project is part of the SPP2225. |
Functional impact of lipid logistics during mycobacteria infection
|
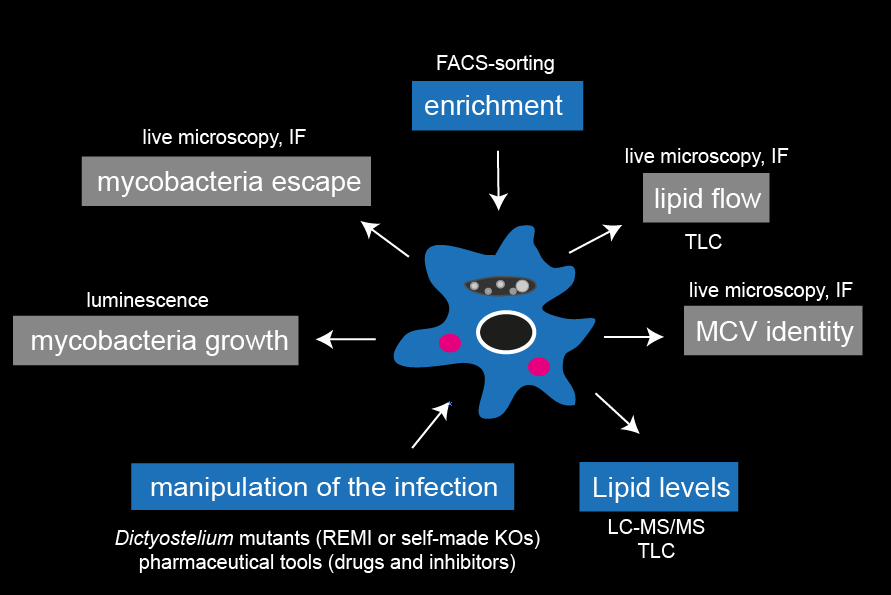
This project aims to identify lipid metabolic pathways that are hijacked by intracellular mycobacteria to exploit lipids from the host. To monitor alterations in lipid levels, we are establishing mass spectrometry lipidomics and thin layer chromatography for the D. discoideum/M. marinum system. In the future, we will determine the consequences of blocking specific lipid supply routes on various stages of the mycobacterial infection course. Collectively, these efforts may uncover novel therapeutic targets to fight mycobacteria infection. Image: Tools to analyse the inhibition of host-to-pathogen lipid flows. |
Induction of membrane contact sites during mycobacteria infection
|
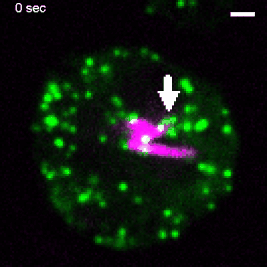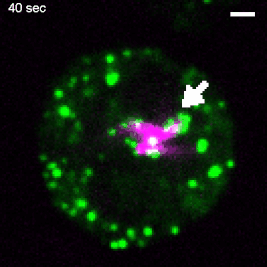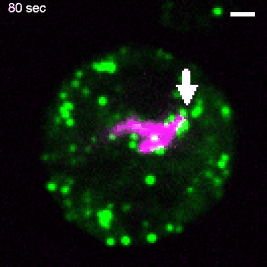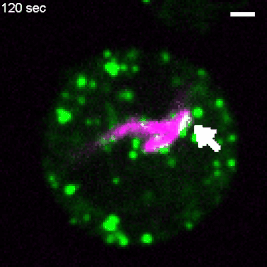
Various intracellular pathogens, including M. tuberculosis, damage the membrane of their vacuoles to impair fundamental innate immune functions and to trigger their translocation into the host cytosol. The host counteracts membrane damage by recruiting membrane repair machineries to retain the pathogen inside the vacuole. Using advanced imaging approaches, the lab investigates the role of ER-dependent membrane repair and other repair machineries during mycobacteria infection. For example, to investigate the formation of membrane contact sites between the ER and the Mycobacterium-containing vacuole (MCV), we employ advanced imaging techniques. Specifically, we utilize several 3D-CLEM approaches that include high-pressure freezing and TEM-tomography (as described in Franzkoch and Anand et al., 2023, BioRxiv) as well as serial block-face SEM (Anand et al., 2023, BioRxiv). This, together with spinning disc live cell imaging and flow cyometry, uncovered that ER-dependent repair constitutes a host defence mechanism against intracellular pathogens such as M. tuberculosis (Anand et al., 2023, BioRxiv). This project is part of the SFB1557 @Uni Osnabrück. |